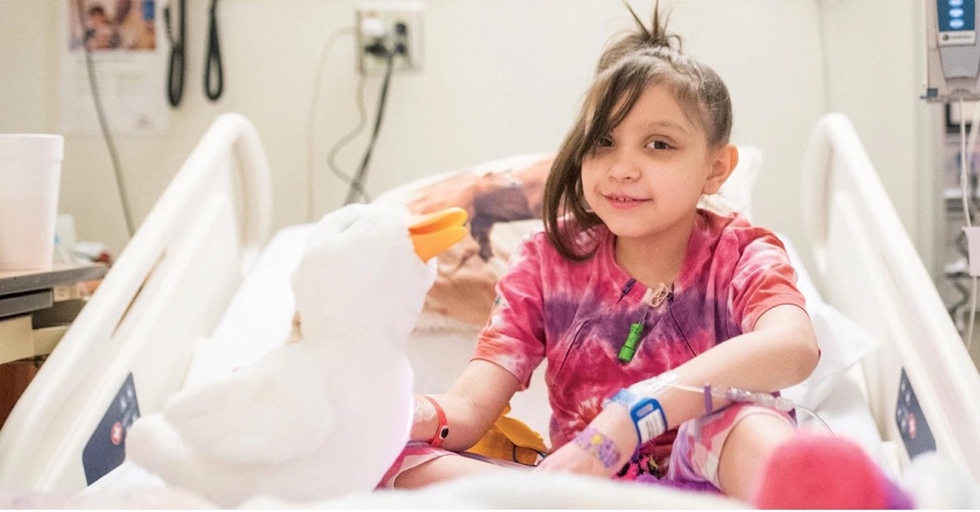
R. Todd Ruppert is the founder and CEO of Ruppert International, a firm with diversified interests globally in various fields including education, financial services, disruptive technologies, arts & entertainment, and strategy consulting. He is chairman of RSR Partners, The World Trade Center Institute, and London's Royal Parks Foundation (USA). He is a
global ambassador for The Duke of Edinburgh's International Award, and a board member of Afriex, Antler, BoxMedia,
Credrails, Culture3, Spod, Hitlab, mPokket, Peko, Pirkx, Shetland Space Centre, Storelli Sports, The International Centre for Missing and Exploited Children, Trinity Street Asset
Management, and The Rock and Roll Hall of Fame. He was a board member of INSEAD Business School from 2012-2021 and currently is a member of its Advisory Council.
Todd is an advisor to fintech advisory firm SenaHill. He is also an advisor to a number of VC/PE firms including Access Holdings, Antler, Balerion Space Ventures, Broadlight Capital,
CXO Fund, Fin Capital, Global Ventures, Launch Africa Ventures, Modi Ventures, Pact VC, Samos Ventures, Sure Ventures, Thirdbase Capital, and Unicorn Growth.
Todd has over 40 years of experience in the financial services industry. He retired from T. Rowe Price, the global asset management firm with over $1.5 trillion under management,
where he was CEO and president of T. Rowe Price Global Investment Services, co-president, T. Rowe Price International,
and a member of the operating steering committee of the T. Rowe Price Group. He also retired from Greenspring Associates, a $17 billion venture capital firm, where he was a venture partner.
His numerous advisory board roles are for the following organizations located around the globe:
Education: ActivEd, Duke University, MarcoPolo Learning, MPOWER
Financing: Financial Services/Disruptive Technologies: Behaviour Lab, Bite Investments, Fiduciary Investors
Symposium, GIST Impact, IMMO, ManageMy, Minimum, Molten Cloud, Moneyhub, Money360, Octopus Investments,
Opto Invest, PivotalPath, Q Ventures, Republic, Trove Arts and Entertainment: Bottletop, Flawlessai, MTArt Agency
Strategy Consulting: Gold Mercury International, Laurel Strategies
Other: Alta, lperionX, PowerX
Todd is the executive producer of three documentary films - A
Year in Burgundy, A Year in Champagne & A Year in Port.